(3) Important factors other than “nutrients”
Emeritus Prof. Yoh Yamashita
PDF is here
日本語の連載はこちら
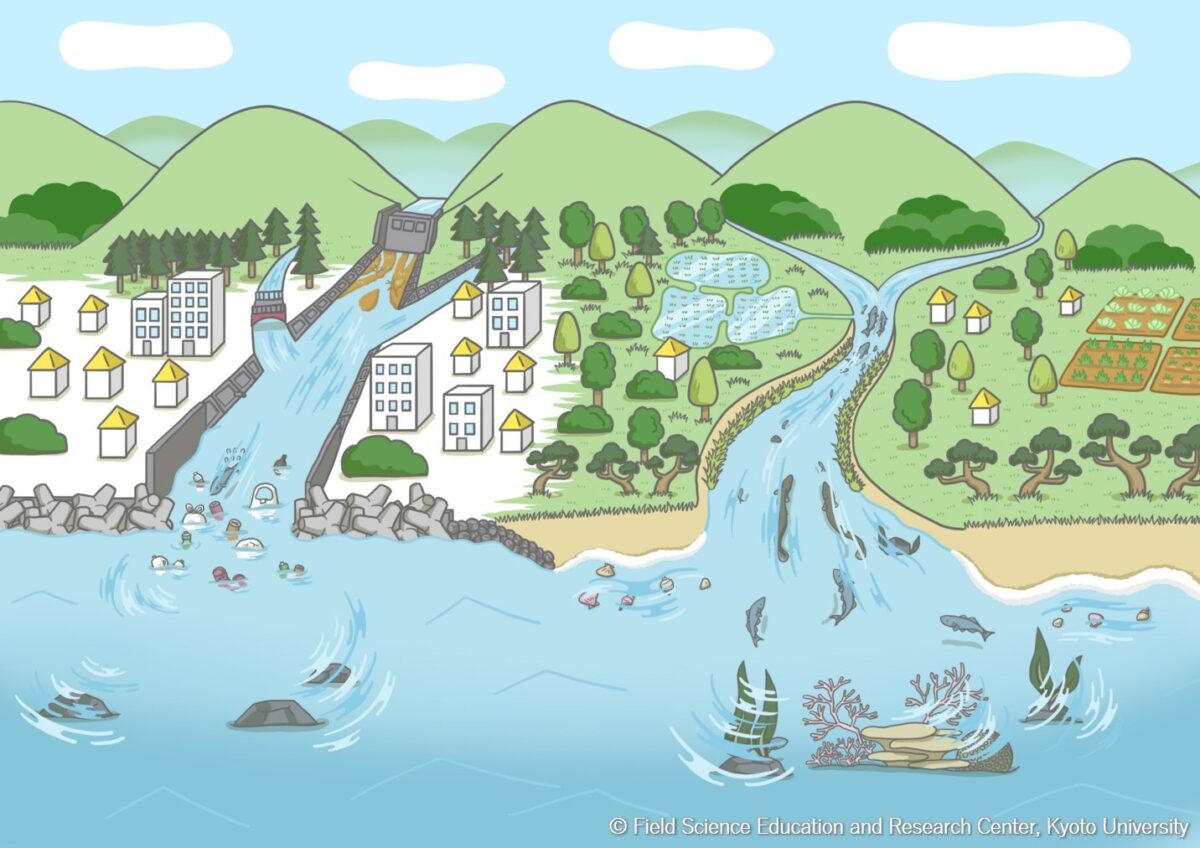
The players connecting forests and seas are many and complex, including aquatic organisms, river water volume, inorganic particles (silt/clay to boulder), organic matter, pesticides and other toxic substances, and artificial rehabilitation of rivers.
In the second article of the Introduction to the CoHHO, I focused on “nutrients” supplied from land to the sea, based on the catchphrase, “Rich nutrients from forests nurture productive coastal seas.” This time, I would like to talk about important factors other than nutrition.
1. Estuarine and coastal food webs and utilization of organic matter
Organic matter produced on land and transported to the sea through rivers includes trees, grass, animal carcasses, discarded human food, sewage, and many others. Organic matter is broken down or decomposed during transport through rivers. It is carried to the sea in a variety of forms, ranging from ultrafine particles dissolved in water (elements that pass-through pore sizes of 0.45 to 1 µm), called dissolved organic matter, to solid organic matter that remains in its original form, such as plant fragments. When organic particles and solid organic matter accumulate on the seafloor, they are decomposed by microorganisms and cause hypoxic conditions. Previously, there were no methods to investigate the role of such diverse organic matter in food webs of rivers and seas. However, recent advances in stable isotope analysis of carbon, nitrogen, and other elements have enabled us to analyze the origin of organic matter and the dietary relationships of the animals that use it. This has led to dramatic advances in research on the actual utilization of organic matter by aquatic animals. The authors have been conducting a detailed investigation of the supply of organic matter from rivers and its utilization by animals in Yura River and Tango Bay in northern Kyoto Prefecture. I would like to introduce some of the results in the following sections.
In Yura River, physical and biological processes can be broadly classified into two major periods. The high discharge period (December to March) is in winter when rain and snowfall causes a large volume of discharge, and the river fills with fresh water to its mouth. The low discharge period is from spring to autumn (April to November), when the river forms a stratified estuary where seawater intrudes as a salt wedge along the river bottom (Figure 1). From early summer to autumn, seawater intrudes up to about 18 km from the river mouth in the downstream and estuarine areas. The distance of seawater intrusion is determined mostly by river discharge and sea level which is higher during the summer (Kasai et al. 2010). During the low discharge period, brackish and marine phytoplankton actively undertake primary production in the lower reaches using abundant nutrients in the river water. A distinct chlorophyll maximum forms in the halocline layer, which appears between the upper and lower layers. During the high discharge period, phytoplankton density becomes extremely low in the river. Contrastingly in Tango Bay, phytoplankton blooms usually occur from February to April owing to nutrients supplied not only from the river but also from offshore through estuarine circulation (see Kasai et al. (2010), Watanabe et al. (2014) and “Introduction to the CoHHO (2)” for details).
We examined the dietary relationships between 135 species of benthic animals (macrobenthos) from the lower reaches to the offshore waters of Yura River using stable isotope analysis of carbon and nitrogen (Antonio et al. 2010a, 2012). We considered five types of organic matter sources as the basis of the food web: terrestrial plants, river particulate organic matter, macroalgae, benthic microalgae, and marine particulate organic matter. River particulate organic matter includes freshwater phytoplankton and broken fragments of terrestrial plants, and marine particulate organic matter consist of mainly of phytoplankton. In the downstream and estuarine areas during the spring and autumn seasons, when seawater intrudes upstream, the analysis showed that various types of organic matter was used as food for benthic animals, from terrestrial plant-derived organic matter to marine phytoplankton (Figure 2). High utilization of terrestrial plant-derived organic matter was found in the lower reaches mainly in winter when discharge is high. In contrast, in the coastal and offshore areas, there was little utilization of terrestrial plants and river particulate organic matter, and the contribution of marine phytoplankton increased with water depth.
Terrestrial plant-derived organic matter contains refractory substances such as cellulose and lignin. It is known that these substances can only be used by animals with cellulase that breaks down cellulose. In Yura River, cellulase activity was observed in the estuarine bivalve Corbicula japonica and estuarine snails Cipangopaludina japonica, Semisulcospira libertina, and Clithon retropictus (Antonio et al. 2010b). These shellfish fed heavily on terrestrial plant-derived organic matter in rivers, whereas in the sea they preferred benthic microalgae- or phytoplankton-derived organic matter to terrestrial plant-derived organic matter. Although human preferences are not directly comparable, I can understand shellfish’s desire to eat seaweed if seaweed and leaves are placed on the same dinner table.
The amphipod Anisogammarus pugettensis, which is abundantly distributed in estuarine areas in Hokkaido, chomps on and digests leaf litter deposited along the coast (Sakurai & Yanai 2008). In comparison, only a limited number of animals feed on terrestrial plant-derived organic matter in the sea, and terrestrial plants which are transported and deposited on the seafloor are mainly decomposed by microorganisms. This has important implications for river management. Natural rivers usually meander and are composed of riffles and pools. It is thought that organic matter of terrestrial origin accumulates in river pools and is decomposed and removed by animals that have cellulases to utilize it. However, rivers are often straightened and modified to have a riffles-only structure with three-sided embankments to prevent flooding. In such rivers, terrestrial plant-derived organic matter is inevitably carried to the sea in a short time and deposited on the bottom of estuaries and inner bays. Since only a limited number of benthos feed on these organic materials, it can be inferred that many of them are decomposed by microorganisms, which in turn consume oxygen and form hypoxic waters. In addition to providing habitats for river animals, meanders and riffle-pool sequences in rivers play an important role in conserving coastal environments.
2. Influence of inorganic particles such as sediment
Various human activities on land brings in a large influx of sediment into rivers. Sediment is supplied from various places, including degraded forests, arable lands (agriculture not used for rice cultivation), rice paddies, construction works, and river improvement works. On the other hand, the influx of sediment into seas greatly decreased after World War II, as many dams were constructed in rivers and sediment accumulates in dams. In Japan, the average annual rate of sediment deposition in dams is estimated to be 1.1%, which means that dams will be buried in about 90 years. Sandy beaches have been receding on most of our nation’s coasts due to a lack of sediment supply (Unoki 2015). In Tenryu River, for example, it is estimated that about 90% of the annual supply of beach-forming sediment has been lost due to dams and gravel extraction from riverbeds (Uda 2010). The recession of sandy beaches and the reduction of tidal flats due to the decline in sediment supply is also thought to be directly related to the decrease in the number of aquatic animals that live or breed in these areas. To prevent the recession of sandy beaches, many beaches in Japan have piled up breakwater concrete blocks to prevent erosion offshore. The typical landscape of Japan, beaches with white sand and green pines, are disappearing from this country.
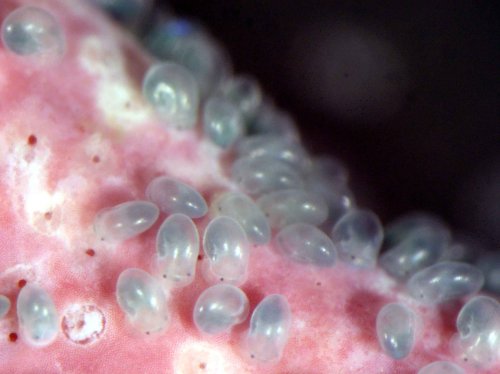
On the other hand, there is concern in recent years about the mud (silt and clay) deposition in rivers and coastal areas. As mentioned above, sediment supply to the sea is declining due to dams. When it rains, only fine particles of less than 50 μm (referred to as “microscopic particles” hereafter) in the sediment of the dam flow out and accumulate on the river bottom and seafloor. Microscopic particles adversely affect aquatic ecosystems such as rivers, tidal flats, beaches, rocky reefs, and coral reefs (Yamashita 2014). There are two ways they can do this. The first is the effect of microscopic particles suspended in water. Microscopic particles can enter the gills of fish and shellfish, causing inflammation, and in some instances, high concentrations have been reported to kill or harm fish. Microscopic particles also increase mortality rates of algal zoospores, sea urchin and abalone larvae, and turbidity interferes with photosynthesis of seaweed and sea grass. The second is the effect of microscopic particles deposited on the bottom. It is well known that microscopic particles destroy coral reef ecosystems (Rogers 1990). In seaweed beds and rocky reefs, even a very thin layer of microscopic particles deposited on the substrate can inhibit zoospore attachment, gametophyte growth, survival, and maturation of seaweed (Matsumoto et al. 2020). In addition, planktonic larvae of reef-dwelling snails, such as turban shells and abalone, selectively settle on fine seaweed called coralline algae (calcareous algae) and use it as a nursery ground during the early juvenile stage (Figure 3). Coralline algae have been reported to emit chemicals that attract these snail larvae and promote settlement and metamorphosis. However, the metamorphosis of abalone planktonic larvae into juvenile snails is severely inhibited when just a thin layer of microscopic particles is deposited on top of the coralline algae (Onitsuka et al. 2008). An increase in mud content in the bottom also creates an unsuitable environment for edible bivalves such as Manila clam (Ruditapes philippinarum) and fan mussel (Atrina pectinata), as well as for juvenile benthic fishes that grow on sandy/muddy bottoms in shallow waters, including Japanese flounder (Paralichthys olivaceus), stone flounder (Platichthys bicoloratus), and marble flounder (Pseudopleuronectes yokohamae) (e.g. Ellis et al. 2002, Yamashita 2014). In addition, the accumulation of microscopic particles containing organic matter on the seafloor cause hypoxic conditions and facilitate the formation of highly biotoxic substances such as hydrogen sulfide. These numerous examples show that microscopic particles are a serious destroyer of coastal ecosystems.
I have mentioned that degraded forests, arable lands, rice paddies, construction works, and river improvement works can be the source of sediment containing microscopic particles. The flow of sediment into the sea through rivers is a natural process that has continued throughout the long history of Earth. Basically, river sediment flowing into the sea in its original form and composition does not cause any problems. However, the sudden appearance of dams in the 20th century physically broke the connectivity between land, rivers, and the sea. Sediment flowing into rivers are deposited in dams, and only the finest particles of the sediment are washed downstream and carried to the coast during high discharge. In other words, sand-sized particles necessary to form sandy beaches are deposited in dams, while only microscopic particles (silt and clay components) are discharged. This is thought to have a significant impact on the bottom environment of rivers and coastal areas.
Although sediment deposition and microscopic particle discharge in dams affect aquatic ecosystems profoundly, I would like to consider other sources of sediment supply. The first is degraded forest plantations. During Japan’s postwar recovery, natural forests were cleared and replaced by plantations on a large scale. Plantations now account for 40% of the country’s forests. However, with the subsequent import of cheap foreign timber, the domestic forestry industry lost its competitiveness and fell into an extreme depression. Forestry workers do not have the capacity to care for plantations, and vast areas of plantations are severely degraded. In these forests that have not been properly pruned and thinned, light does not penetrate within and the understory vegetation does not form. This leaves the soil bare, and when it rains, the topsoil runs off into rivers (Onda 2008).
Arable land (farmland) and rice paddies are also considered to be important sources of microscopic particles (Nishimura 2011, Nunes et al. 2011). The efflux of sediment is determined by the extent of vegetation coverage on soil surfaces. In particular, arable land is bare soil, with the exception of pastures, and much of it is eroded by rain and washed into rivers. Soil erosion in arable land not only causes loss of soil and fertilizer components, but also poses a serious problem as a non-point source of river water pollution. To prevent soil and fertilizer components from flowing into rivers, measures are being taken such as sedimentation ponds and vegetation strips at boundaries of farmlands. In our study, we found a negative relationship between the diversity of endangered fish species in estuaries and the area of arable land in their watersheds. One possible cause of the negative impact of arable land is the inflow of microscopic particles into rivers (Lavergne et al. 2022).
Paddy fields are another important source of soil (Nishimura 2011). In the postwar period, intake and drainage routes were separated in the irrigation system of paddy fields by the government’s agricultural development program. In old paddy fields, suspended particles from plowing were deposited in the paddies downstream, but after infrastructure improvement, turbid water is discharged from individual paddies directly into rivers (Figure 4). Sediment also flows into rivers from abandoned farmland, which has been increasing in recent years due to acreage reduction policy of the Ministry of Agriculture and the retirement of farmers. It is known that re-vegetation of such abandoned farmland (conversion to grassland or woodland) can reduce the degree of topsoil erosion by two to three orders of magnitude. Another possible factor is the influx of sediment from cities and construction sites. In Okinawa, civil engineering and development works are considered the main cause of red soil runoff that destroys coral reefs.
3. Toxic substances flowing into the sea
Pesticides such as herbicides, fungicides, and insecticides represent toxic substances that flow into the sea from land. Since these chemicals are designed to kill weeds and insects, they are bound to be harmful to humans and ecosystems. However, pesticides are far from my expertise, and I will not discuss in detail here.
The use of pesticides spread rapidly in Japan after World War II. Harmful chemicals that even killed fish, such as mercury, parathion, DDT, and PCP, were commonly used in fields until the 1960s. At that time, not much thought was given to the negative effects on human health, let alone ecosystems. Rachel Carson’s “Silent Spring” sounded a strong alarm about this problem. It is said that the book drew worldwide attention to the dangers of pesticides, which in turn led to research and use of safer pesticides. Even though the dangers have subsided, these chemicals may still have significant adverse effects on humans and ecosystems because they are designed to kill living creatures. Dioxin, which is known to be the strongest poison ever created by mankind, was used for pesticides in Japan until the mid-1980s. Moreover, TeCDD, a representative of more than 200 substances included in dioxin, has a relatively long half-life of 8.7 years. Therefore, it may still be flowing into coastal areas from fields through rivers and depositing on the bottom of enclosed bays (Okumura et al. 2004).
The mainstream insecticides used today are called neonicotinoids. Their principle use in Japan is to kill stink bugs and other insects that suck rice juice from husks and cause spots on rice. However, these pesticides are so persistent that they remain in pollen and in the hydrosphere for long periods. As a result, they end up killing many insects including aquatic ones, and are said to be the cause of a drastic decrease in honeybees and dragonflies in recent years. In 2019, a paper was published indicating that neonicotinoid pesticides destroyed the aquatic ecosystem in Lake Shinji, causing a sharp decline in Japanese eel (Anguilla japonica) and Japanese smelt (Hypomesus nipponensis) (Yamamuro et al. 2019). While it is said that they have little effect on humans, some argue that it causes neurodevelopmental disorders, and no conclusion has been drawn yet. EU has banned three of the five major neonicotinoid pesticides and France has banned their use altogether. In contrast, Japan continues to take measures that are completely opposite to such global trends by expanding the application of neonicotinoid pesticides and relaxing food residue standards. Speckled rice is a serious problem for farmers as the spots reduce its grade, but the taste is thought to be unaffected. There are people, especially from overseas, who argue that we are endangering our ecosystem and human health just to eliminate these spots. I believe this is a typical problem of “individual optimization”, and the concept of “overall optimization” is essential for SDGs, mentioned in the first article of this series.
There are many other substances that disturb ecosystems through human activities, such as mercury, PCBs that were used in industrial products, cadmium that originated from mines accumulated in paddy fields, and chemicals that received attention as environmental hormones. However, evaluation of their impact on ecosystems has not progressed a lot.
4. Production, growth, and movement of animals in rivers and estuaries
Migration of aquatic animals between rivers and the sea is called diadromous migration, and it is categorized into three types: catadromous migration, where aquatic animals grow in rivers and descend to the sea to spawn (e.g. eels); anadromous migration, where aquatic animals grow in the sea and return to rivers to spawn (e.g. salmons); and amphidromous migration, where aquatic animals move between rivers and the sea without being directly related to spawning (e.g. Ayu sweetfish, Plecoglossus altivelis). We have been studying how amphidromous temperate seabass (Lateolabrax japonicus) and catadromous Japanese eel (Anguilla japonica) utilize rivers and the sea. Here, I would like to share the results with a focus on the role of the estuarine area and explain it in terms of the CoHHO.
(1) Production of juvenile seabass
Yura River, which appears frequently in this article, is a first-class river flowing through the northern part of Kyoto Prefecture with a main channel of 147 km, a basin area of 1882 km2, and a basin population of approximately 170,000 people. Yura River originates in Ashiu Research Forest, Field Science Education and Research Center, Kyoto University. It flows into the innermost part of Tango Bay, about 30 minutes by car from Maizuru Fisheries Research Station, Kyoto University. Because of its location, Yura River has been the most important field for the Field Science Education and Research Center’s CoHHO research. The shallow waters of Tango Bay are the nursery grounds for important aquatic animals in fishery, such as temperate seabass, Japanese flounder, squids, and sparid and carangid fishes. Many of the juvenile fish in this area feed mainly on a group of small crustaceans called mysids.
Temperate seabass spawns offshore in Tango Bay from December to February, and the hatched larvae, a few millimeters long, are transported to the coast using shoreward currents created by strong northwesterly winds in winter. In February and March, they settle down on the seafloor about 10 m deep offshore of the mouth of Yura River (Suzuki et al. 2020). In mid-April, the decline of melting snow decreases the discharge of Yura River, and juvenile fish distributed off its mouth enter the river with intruding seawater. We found that temperate seabass juveniles that move into Yura River migrate as far as 50 km upstream from the mouth of the river. Once in the river, they remain there until around autumn.
On the other hand, since a large number of temperate seabass juveniles continue to inhabit the shallow waters of Tango Bay after April, it is thought that the juveniles that migrate up Yura River are a fraction of the juveniles that arrive at the river mouth in February and March. To investigate the benefits of upstream migration, we compared the food intake and growth rate of juveniles collected in Yura River and Tango Bay. The results showed that juveniles in the river fed more and grew better than those in the sea (Figure 5). The primary food source of temperate seabass juveniles is mysids, which are abundantly distributed in the coastal area from February to June (mainly Orientomysis japonica) and in the lower reaches of the river from May to August (mainly Neomysis awatschensis). In other words, temperate seabass lives in shallow waters of the coastal area until March, where Orientomysis japonica are abundant, and some move to the lower reaches of Yura River from April/May to feed on the dominant estuarine mysid Neomysis awatschensis. This indicates that temperate seabass effectively utilizes the productivity of both the lower reaches of the river and the coastal area (Kasai et al. 2018). Given that the coastal nursery is much larger in area, the large proportion of juveniles is thought to remain in the sea.
To investigate the contribution of rivers and the sea as nursery grounds for juvenile seabass, we analyzed trace elements (ratio of strontium Sr to calcium Ca) in otoliths, the only hard tissue in fish that is not metabolized (its components are not replaced like regular bones). Strontium is abundant in sea water, but only a small amount is found in river water. Therefore, by examining the Sr:Ca ratio of otoliths formed during the juvenile stage, we can determine the juvenile’s experienced environment (river or sea). Using this method, we examined otoliths of temperate seabass adults caught by set nets in Tango Bay and found that nearly half of the juveniles had a history of using rivers as nursery grounds (Fuji et al. 2016). In addition, it is possible to estimate the daily growth history of each fish from hatching to capture based on the analysis of daily rings inscribed in otoliths, since the intervals between the rings are proportional to daily growth (Figure 6). This technique also revealed that, among the juveniles that had stayed in the coastal area in February and March, the slow-growing smaller individuals migrated to the river, while the larger ones remained in the coastal area. We also found that the river migrant juveniles fed on an abundance of mysids and grew well, and by the time they returned to the sea in autumn, their length had caught up with the coastal residents (Fuji et al. 2014). The primary diet of juvenile seabass in Yura River is the mysid Neomysis awatschensis. They usually live near the river bottom and feed on attached benthic microalgae. These benthic microalgae are mainly produced by nutrients of terrestrial origin in freshwater areas (Omweri et al. 2021).
Large adults of temperate seabass also migrate up Yura River. Temperate seabass up to 70 cm in length can be caught in mountain streams as far as 40 km upstream from the river mouth. We are currently conducting research on large adults of temperate seabass that migrate up Yura River. In the process, we have discovered that Ayu sweetfish is an important target prey of large adults in the river. Interestingly, Ayu sweetfish feed on the very same attached microalgae produced by nutrients from forests. If the research progresses, we may be able to see how temperate seabass in Tango Bay is nurtured by Tamba Forest (i.e. forests in the central part of Kyoto Prefecture).
(2) Revival of Japanese eel
It is well known that the stock of Japanese eel (hereafter referred to as “eel”) has been drastically depleted. Although the full life-cycle aquaculture (raising fish from fertilized eggs to matured adults) of eel has been successful at the laboratory level, the number of eel that can be produced is extremely limited and we are far from developing a method sufficient for commercial purposes. Therefore, traders catch large quantities of glass eel for aquaculture. Eel larvae (leptocephali) are carried from their spawning grounds on the distant West Mariana Ridge by the Kuroshio Current, before metamorphosing into glass eels in estuarine waters of Japan from winter to spring. Glass eel fishery is far from being managed and overfishing of glass eel is the primary factor in the decline of the eel stocks. Only glass eels that are lucky enough not to be caught change their body color from white to black (i.e. juvenile elver eels). Eels grow in a wide range of habitats from estuaries to rivers. Eels in this stage are called yellow eels because of the yellowish coloration on their bellies. When yellow eels reach age about five for males and age 10 for females, they change into silver eels, and go out to the open sea for spawning migration. The second cause of the decline in the eel stock in Japan is habitat loss due to environmental degradation of rivers and estuaries, as the rivers and estuaries serve as important habitats for wild eels. Here, I would like to discuss this second issue from the viewpoint of the CoHHO studies.
Our research group is conducting ecological study on eels in Oita, Wakayama, and Fukushima Prefectures. We made some important discoveries in this process. Eels are quite tolerant of river water quality, especially organic pollution. In other words, they can live without any problem in both clear and slightly polluted urban rivers, as long as the conditions are within certain acceptable levels. In a study by Kasai et al. (2021), they used environmental DNA in rivers throughout Japan from Okinawa to Hokkaido. They found that rivers with higher concentrations of eel environmental DNA also tended to have higher concentrations of total nitrogen. Total nitrogen concentration is considered an indicator of eutrophication, but recently the quality of river water in Japan has improved substantially compared to the period of rapid economic growth in the 1960s and 70s. Therefore, a river with high total nitrogen concentration can be considered productive and rich today. In the surveys in the three prefectures mentioned above, wild eels were found in many rivers, including clear streams inhabited by chars (Salvelinus spp.) and Yamame trout (Oncorhynchus masou), irrigation canals in paddy fields, and even urban rivers with sewage inflows. Some urban rivers were found to be inhabited by wild eels at densities far exceeding the distribution densities published in academic journals (Kume et al. 2021).
This study revealed several characteristics of rivers where eels can live naturally. First, it is important that there should be no crossing structures (dams, weirs, water gates) that impede their migration, as eels come from the sea and migrate upstream. Yet eels are known to climb weirs tenaciously. We observed that juvenile eels could climb a weir 1.65 m high. Recently, it has been reported that juvenile eels can climb a waterfall of 46 m in height (Matsushige et al. 2021). However, the structure of weir walls is an important factor, and eels cannot climb walls without some irregularities. In addition, long water plants and other vegetation hanging from upstream of weirs play an important role in eels’ ascent. However, even though juvenile eels can climb walls of weirs, there are limits. The number of eels inhabiting rivers decreases with each additional weir upstream, and weirs undoubtedly hinder their distribution (Kume et al. 2019, 2020). Attaching irregularities to the wall of weirs would facilitate climbing by juvenile eels, and therefore, such irregularities would certainly aid their migration. However, it is not useful for the migration of older yellow eels or other fish (Ayu sweetfish, Yamame trout and chars) and it is still important to install passageways that allow these fish to migrate upstream.
We also tracked eels by attaching transmitters to their bodies and examined their feeding habits using stable isotope analysis (Kutzer et al. 2020, Noda et al. 2021). The study found that eels lead a well-regulated life as they used the estuary to roost during the day, entered the river at sunset, fed there during the night, and returned to their roost before sunrise. It is interesting that some of the eels that roosted at the estuary also went seaward to feed at night. Individual eel chooses its feeding ground depending on the spatiotemporal changes in food availability. If rivers and the sea, or rivers themselves, were divided by weirs, it would be difficult for eels to secure sufficient food through such flexible foraging.
In addition to the absence of weirs, the availability of aquatic prey animals and hiding places is also important for eels to live comfortably in rivers. Eels are known as carnivores and they can feed on a variety of animals. Eels feed mainly on aquatic insects until they reach 20 cm in length, and thereafter on shrimp, crabs, and fish. It is also reported that eels feed on earthworms in forest rivers (Itakura et al. 2015). Small eels live under pebbles, while large eels can hide under large stones (stones not buried in the riverbed), among aquatic plants, or in the mud. In other words, eels can live anywhere in a normal river as long as the river has riffles and pools, the riverbed is composed of various substrates from mud to large stones, and if there are plants in the water or along the banks(Kume et al. 2019, 2020). However, many rivers in Japan are managed with a focus on “water utilization” and “flood control”, and little consideration is given to the “environment and biodiversity” which is the other core pillar of the River Law in Japan. Many of the natural rivers with meanders, riffles and pools have been straightened and revetted with concrete to make drainage channels that simply let water flow. Such rivers also have few living creatures. Even in rivers where the natural state has been preserved for eels and Ayu sweetfish to inhabit, heavy machinery is used every year to do civil work on the riverbanks and riverbed. In these rivers, a large amount of soil is washed out every time construction is carried out, covering the microalgae on stones that Ayu sweetfish would otherwise eat. This is one of the reasons for the sharp decline in Ayu sweetfish in recent years. Our river ecosystem is currently being destroyed by construction works for a meaningless measure in the name of reducing unemployment.
Environmental issues such as global warming began to attract attention around the 1980s as a worldwide crisis. However, it is only recently that people have begun to recognize it as a reality in their social lives. The global environment is clearly deteriorating. People are experiencing an increase in extreme weather events such as heat waves, heavy rains, hyper typhoons, and consequent disasters. Population continues to grow on our finite planet and is expected to reach nearly 10 billion by 2050. If we do not recover Earth’s system so that the environment is preserved and resources are recycled, the human race will certainly face a crisis within our lifetime. The CoHHO studies consider the symbiosis between humans and nature scientifically through the restoration of the cycle of nature. In the first article of “Introduction to CoHHO”, I talked about individual optimization and overall optimization. Instead of chasing short-term profits (individual optimization), we need to apply wisdom from a broad and long perspective grounded on science (overall optimization) for the sustainable future of Earth and mankind. The achievement of the SDGs is an issue of personal interest that directly affects our lives.
Key words: food web, fine sediments, pesticides (neonicotinoids), forests, agricultural land, rice paddies, sea bass, ayu, Japanese eel
Reference
Antonio, E. S. et al. 2010a. Spatial variation in organic matter utilization by benthic communities from Yura River-Estuary to offshore of Tango Sea. Estuarine, Coastal and Shelf Science, 86, 107-117.
Antonio E. S. et al. 2010b. Consumption of terrestrial organic matter by estuarine molluscs determined by analysis of their stable isotopes and cellulase activity. Estuarine, Coastal and Shelf Science, 86, 401-407.
Antonio, E. S. et al. 2012. Spatial-temporal feeding dynamics of benthic communities in an estuary-marine gradient. Estuarine, Coastal and Shelf Science, 112, 86-97.
Ellis, J. 2002. Determining effects of suspended sediment on condition of a suspension feeding bivalve (Atrina zelandica): results of a survey, a laboratory experiment and a field transplant experiment. Journal of Experimental Marine Biology and Ecology, 267, 147-174.
Fuji, T. et al. 2014. Growth and migration patterns of juvenile temperate seabass Lateolabrax japonicus in Yura River estuary, Japan -combination of stable isotope ratio and otolith microstructure analyses. Environmental Biology of Fishes, 97, 1221-1232.
Fuji, T. et al. 2016. Importance of estuarine nursery areas for the adult population of the temperate seabass Lateolabrax japonicus, as revealed by otolith Sr:Ca ratios. Fisheries Oceanography, 25, 457-469.
Itakura, H. et al. 2015. Feeding, condition, and abundance of Japanese eels from natural and revetment habitats in the Tone River, Japan. Environmental Biology of Fishes. 98, 1871-1888.
Kasai, A. et al. 2010. Salt-wedge intrusion of seawater and its implication for phytoplankton dynamics in the Yura Estuary, Japan. Estuarine, Coastal and Shelf Science, 86, 408-414.
Kasai, A. 2018. Partial migration of juvenile temperate seabass Lateolabrax japonicus: a versatile survival strategy. Fisheries Science, 84, 153-162.
Kasai A. et al. 2021. Distribution of Japanese eel Anguilla japonica revealed by environmental DNA. Frontiers in Ecology and Evolution, 25. https://doi.org/10.3389/fevo.2021.621461
Kume, M. et al. 2019. Longitudinal distribution and microhabitat use of young Japanese eel Anguilla japonica in a small river flowing through paddy areas. Journal of Applied Ichthyology, 35, 876-883.
Kume, M. et al. 2020. Size-dependent change in habitat use of Japanese eel Anguilla japonica during the river life stage. Environmental Biology of Fishes, 103, 269-281.
Kume, M. 2021. River to river: First evidence of eel movement between distant rivers via the sea. Environmental Biology of Fishes, 104, 529-533.
Kutzer, A. 2020. Foraging behavior of yellow-phase Japanese eels between connected fresh- and brackish water habitats. Environmental Biology of Fishes, 103, 1061-1077.
Matsumoto et al. 2020. Impacts of sub-micrometer sediment particles on early-stage growth and survival of the kelp Ecklonia bicyclis. Scientific Reports, 10, 20689.
Matsushige et al. 2021. Japanese eels, Anguilla japonica, can surmount a 46‑m‑high natural waterfall of the Amikake River system of Kyushu Island, Japan. Ichthyological Research, 68, 21-31.
Nishimura, K. 2011. Agricultural land and watershed environment. In CoHHO Studies: Aiming for Integrated Management from the Forest to the Sea. supervised by Y. Yamashita, Kyoto University Press, Kyoto, 245-251. (In Japanese)
Noda, T. 2021. Migration, residency and habitat utilisation by wild and cultured Japanese eels (Anguilla japonica) in a shallow brackish lagoon and inflowing rivers using acoustic telemetry. Journal of Fish Biology, 98, 507-525.
Nunes, A.N. et al. 2011. Impacts of land use and cover type on runoff and soil erosion in a marginal area of Portugal. Applied Geography, 31, 687-699.
Okumura, Y. et al. 2004. Historical trends of PCDD/Fs and CO-PCBs in a sediment core collected in Sendai Bay, Japan. Water Research, 38, 3511-3522.
Omweri, J. O. et al. 2021. Flexible herbivory of the euryhaline mysid Neomysis awatschensis in the microtidal Yura River estuary, central Japan. Plankton and Benthos Research, 16, 278-291.
Onda, Y. 2008. The Devastation of Artificial Forests and the Actual Situation of Water and Sediment runoff. Iwanami Shoten Publishers, Tokyo, 1-245. (In Japanese)
Onitsuka et al. 2008. Effects of sediments on larval settlement of abalone Haliotis diversicolor. Journal of Experimental Marine Biology and Ecology, 365, 53-58.
Rogers, C.S. 1990. Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series, 62, 185-202.
Sakurai, I. and Yanai, S. 2008. Utilization of forest organic matter by immature flatfish. In ” Linkage of Forest, River and Sea and Biological Production in Estuary and Coastal Area”, edited by Y. Yamashita and K. Tanaka, Koseisha-Koseikaku, Tokyo, 74-88. (In Japanese)
Suzuki, W. K. et al. 2020. Winter monsoon promotes the transport of Japanese temperate bass Lateolabrax japonicus eggs and larvae toward the innermost part of Tango Bay, the Sea of Japan. Fisheries Oceanography, 29, 66-83.
Uda, T. (2010) Japan’s Beach Erosion -Reality and Future Measures. World Scientific Publishing, Singapore.
Unoki, S. 2015. Morikawaumino-suikei-Keiseito-setsudan-no-kyoi. Koseisha-Koseikaku, Tokyo. (In Japanese)
Watanabe, K. et al. 2014. Influence of salt-wedge intrusion on ecological processes at lower trophic levels in the Yura Estuary, Japan. Estuarine, Coastal and Shelf Science, 139, 67-77.
Yamamuro, M. et al. 2019. Neonicotinoids disrupt aquatic food webs and decrease fishery yields. Science, 366, 620-623.
Yamashita, Y. 2014. Present situation and issues regarding the connectivity between terrestrial and coastal ecosystems. In Shimizu N. et al. eds., Connectivity of Hills, Humans and Oceans. Kyoto University Press, Kyoto. 194-208.